What is organic compound?
Life is based on carbon ability
to form diverse structures and an endless number of different carbon based
molecules. The study of carbon containing compound has come to be known as
Organic Chemistry. The food we intake, the fragrance we inhale, the colors we
see are predominantly due to organic compounds. Organic chemistry deals not
only with the chemistry of life and the natural carbon compounds but also with
the huge, increasing number of synthetic carbon compound. There is hardly any
walk of life where we do not need the organic compounds. Plastic is one of the
instance of organic compound.
Forty years ago, anything made of
plastic was considered "cheap." That’s certainly not true today when
plastics are used in thousands of products ranging from computers, automobile
parts and important medical equipment to toys, cookware, sports equipment, and
even clothes. And the plastics industry continues to grow rapidly.
Introduction of plastic
The first important plastic, celluloid which is a mixture of cellulose nitrate,
camphor, and alcohol and is thermoplastic. However, plastics did not come into
modern industrial use until after the production of Bakelite . Bakelite, is made by the polymerization of
phenol and formaldehyde through thermosetting.
.
How polymerization
occur?
Plastics are synthetic materials, which means that they are
artificial, or manufactured. The building blocks for making plastics are small organic molecules
- molecules that contain carbon along with other substances. They generally
come from oil (petroleum) or natural gas, but they can also come from other
organic materials such as wood fibers, corn, or banana peels. Each of
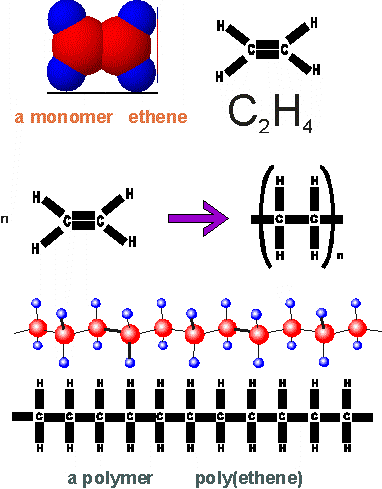
these small molecules is known as a monomer ("one part") because it's capable of joining
with other monomers to form very long molecule chains called polymers("many
parts") during a chemical reaction called polymerization. Polymerization is often started by combining the monomers
through the use of a catalyst - a substance that aids a
chemical reaction without undergoing any permanent chemical change itself.
1. Crude oil, the unprocessed oil
that comes out of the ground, contains hundreds of different hydrocarbons, as
well as small amounts of other materials. The job of an oil refinery is to
separate these materials and also to break down hydrocarbon into smaller one.
2. A petrochemical plant receives
refined oil containing the small monomers they need and creates polymers
through chemical reactions.
3. A plastics factory buys the end
products of a petrochemical plant - polymers in the form of resins - introduces
additives to modify or obtain desirable properties, then molds or otherwise
forms the final plastic products.
An example of polymerization,
production of nylon-6,6
Structure
of polymer
There are polymers that contain
only carbon and hydrogen (for example, polypropylene, polybutylene,
polystyrene, and polymethylpentene). Even though the basic makeup of many
polymers is carbon and hydrogen, other elements can also be involved. Oxygen,
chlorine, fluorine, nitrogen, silicon, phosphorous, and sulfur are other
elements that are found in the molecular makeup of polymers. Polyvinyl chloride
(PVC) contains chlorine. Nylon contains nitrogen and oxygen. Teflon contains
fluorine. Polyesters and polycarbonates contain oxygen. Vulcanized rubber and
thiokol contain sulfur. There are also some polymers that, instead of having
carbon backbones, have silicon or phosphorous backbones. These are considered
inorganic polymers. One of the most famous silicon-based polymers is Silly
PuttyTM
Enviromental issue
Plastic waste in Pacific Ocean
Marine life will be threaten if wastage of plastic did not solve in the future.
Plastics
are durable and degrade very slowly; the chemical bonds that
make plastic so durable make it equally resistant to natural processes of
degradation. Since the 1950s, one billion tons of plastic have been discarded
and may persist for hundreds or even thousands of years. Perhaps the biggest
environmental threat from plastic comes from nurdles, which are the raw material from which
all plastics are made. They are tiny pre-plastic pellets that kill large
numbers of fish and birds that mistake them for food. Prior to
the ban on the use of CFCs in extrusion of polystyrene the
production of polystyrene contributed to the depletion of the ozone layer; however, non-CFCs are currently used
in the extrusion process. Nowdays,
there are quite often the nation concern about this environmental issue. Most
of the country are go towards protecting the environment by using biodegrable
bag instead of the plastic. This is why many slogan were being launch such as ‘Go Green’
Alertness of public to use recycle bag rather than plastic bag.
1.5 tonne of plastic bottles are produced annually, it is the time to Go Green!
A 6 year-old child also knew what are '3R' stands for, do you know that?
Is it impossible for human live without plastic products? As we insist on to be changed, we can do it!
 |
Does it shocked you? New era of '4R' , refill the plastic bottles rather pay for another plastic bottles.  |














No comments:
Post a Comment